FDA Registered
503B Outsourcing Facility
For the best in pharmaceutical compound manufacturing,
trust the art and science of professional compounding to the experts at FarmaKeio Outsourcing.
Our Mission.
To positively impact people’s lives by providing safe, effective and accessible compounded medications.
Quality.
FarmaKeio Outsourcing LLC shall comply with all state and federal regulations regarding compounding medications in an Outsourcing Facility as defined by the FDA in section 503B of the Federal Food, Drug and Cosmetic Act. FKO’s executive management shall maintain and continually work to improve a robust Quality Management System (QMS), which outlines and defines expectations with regards to quality in the organization.
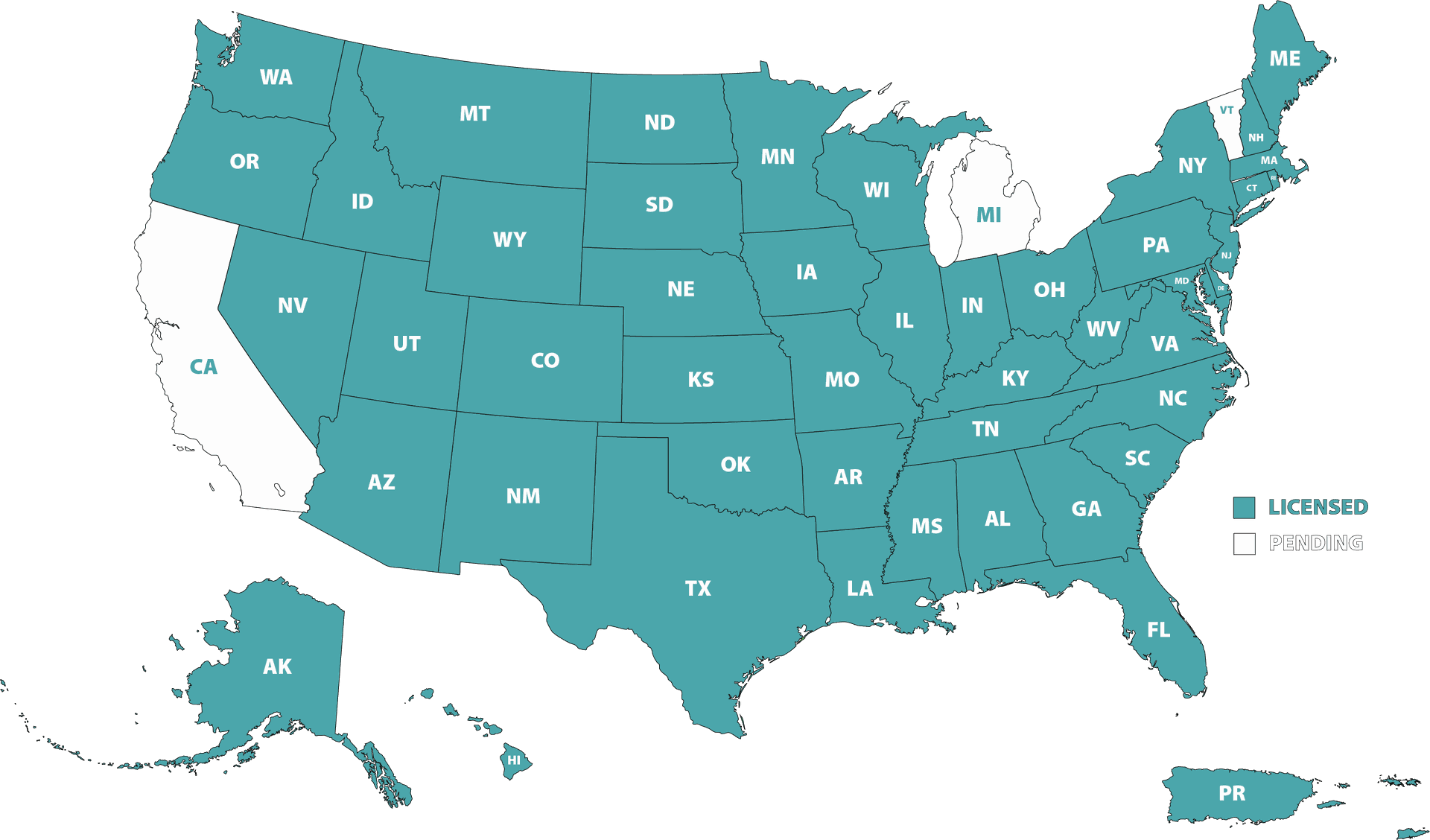
Where We Are Licensed